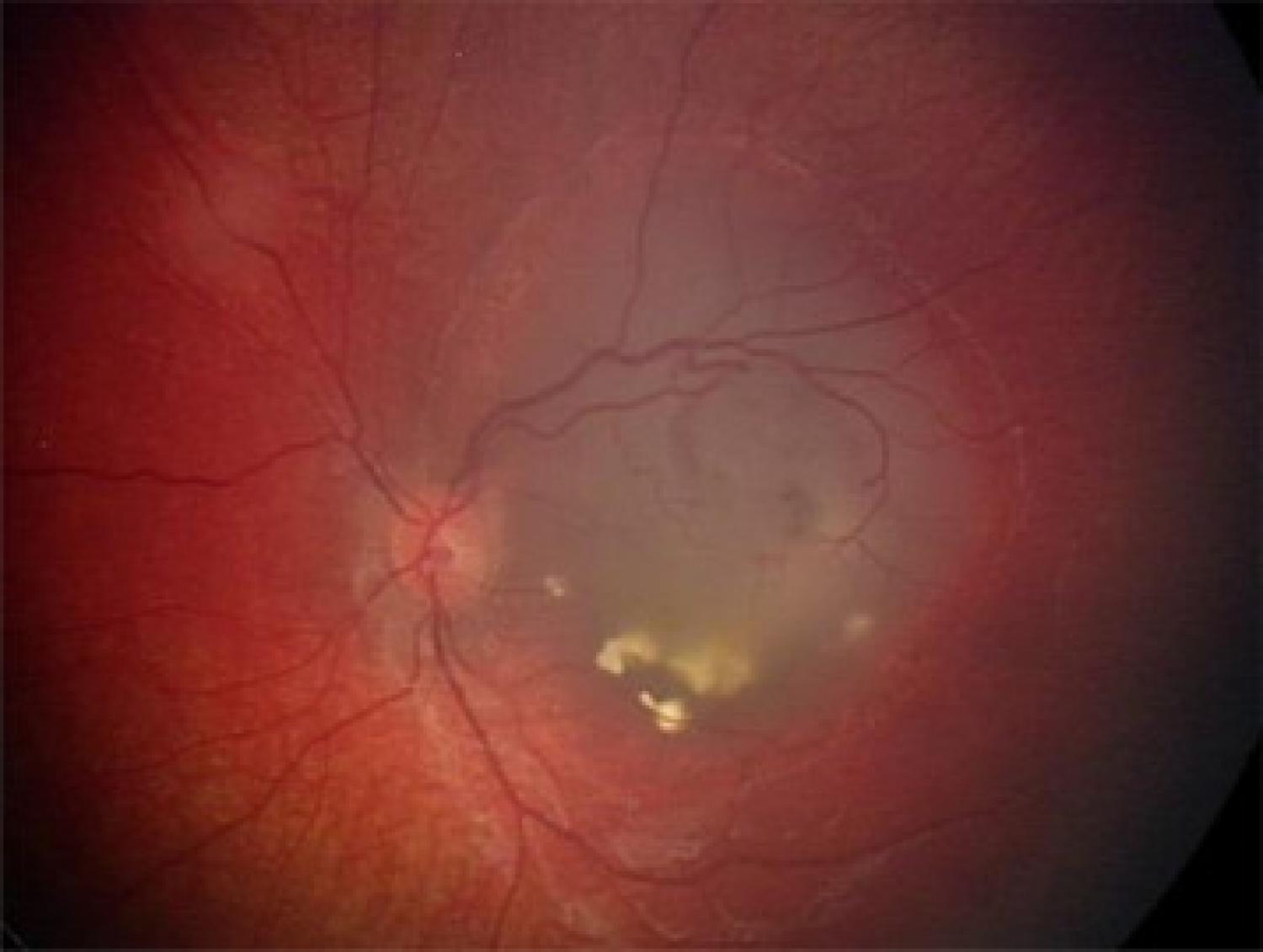
Retinoblastoma is the commonest primary malignant intraocular tumour of childhood accounting for ~1% of tumours in infancy. In most cases, it arises as a consequence of the silencing/loss of both copies of the tumour suppressor gene RB1 in developing retinal cells. The incidence of sporadic retinoblastoma is 1 in 15, 000–20,000 live births, with no gender or racial predilection. The median age at presentation is under 12 months in heritable cases (i.e. individuals with a family history of the condition), and closer to 24 months in sporadic cases. Presentation after the age of 6 years is extremely rare, although there are isolated reports of cases presenting as late as 26 years. Heritable retinoblastoma is prototypical of a cancer susceptibility syndrome. Affected individuals carry a single pathogenic copy of the RB1 gene that predisposes to retinoblastoma formation. All individuals with bilateral retinoblastoma carry such a pathogenic RB1 allele. Importantly, they also have a risk of developing other cancers and lifelong surveillance is required. Nearly 20% of individuals with unilateral retinoblastoma carry a pathogenic RB1 allele that can be transmitted to their children. For a unilaterally affected person with no detectable pathogenic allele with high sensitivity testing, the chance of having heritable retinoblastoma is <1%. Early recognition and diagnosis, alongside effective/optimised multidisciplinary care, results in a 95% cure rate for affected individuals. By contrast, delayed diagnosis results worldwide in around 70% mortality. Understanding the genetics of retinoblastoma has enabled clinicians to develop targeted screening guidelines based on genetic risk, minimising unnecessary screening exams, and focusing resources on individuals at greatest risk.