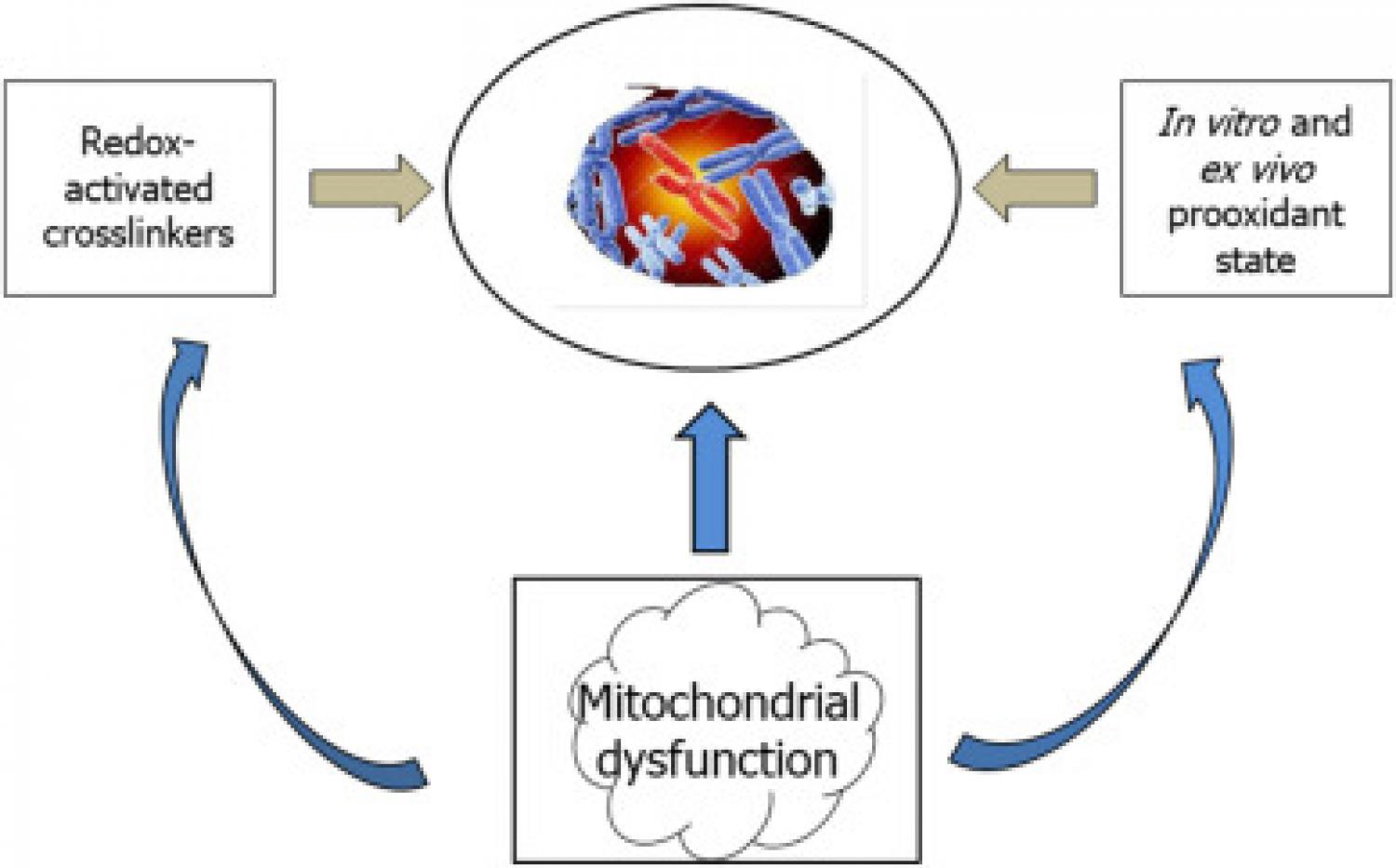
Fanconi anemia (FA) has been investigated since early studies based on two definitions, namely defective DNA repair and proinflammatory condition. The former definition has built up the grounds for FA diagnosis as excess sensitivity of patients’ cells to xenobiotics as diepoxybutane and mitomycin C, resulting in typical chromosomal abnormalities. Another line of studies has related FA phenotype to a prooxidant state, as detected by both in vitro and ex vivo studies. The discovery that the FA group G (FANCG) protein is found in mitochondria (Mukhopadhyay et al., 2006) has been followed by an extensive line of studies providing evidence for multiple links between other FA gene products and mitochondrial dysfunction. The fact that FA proteins are encoded by nuclear, not mitochondrial DNA does not prevent these proteins to hamper mitochondrial function, as it is recognized that most mitochondrial proteins are of nuclear origin. This body of evidence supporting a central role of mitochondrial dysfunction, along with redox imbalance in FA, should lead to the re-definition of FA as a mitochondrial disease. A body of literature has demonstrated the beneficial effects of mitochondrial cofactors, such as α-lipoic acid, coenzyme Q10, and carnitine on patients affected by mitochondrial diseases. Altogether, this re-definition of FA as a mitochondrial disease and the prospect use of mitochondrial nutrients may open new gateways toward mitoprotective strategies for FA patients. These strategies are expected to mitigate the mitochondrial dysfunction and prooxidant state in FA patients, and potentially protect transplanted FA patients from post-transplantation malignancies.